The Innovative Health Initiative (IHI) is funded jointly by the European Union (represented by the European Commission) and the life science industries (represented by COCIR, EFPIA / Vaccines Europe, EuropaBio, MedTech Europe).
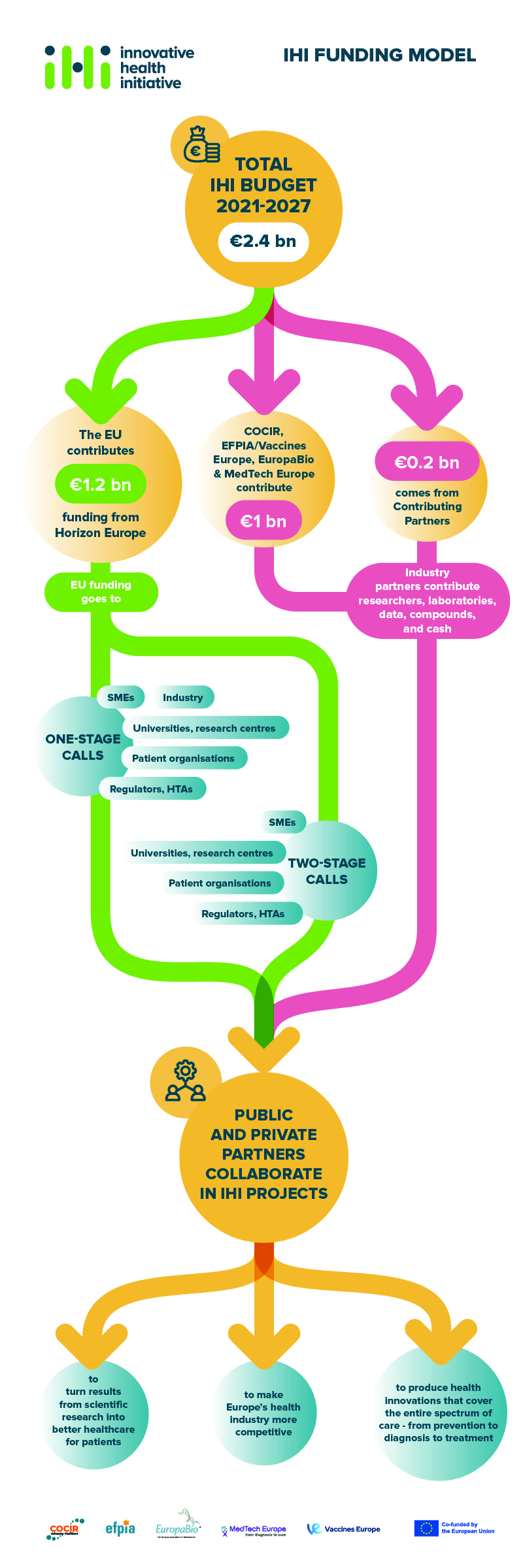
The total budget for IHI for the period 2021-2027 is €2.4 billion.
- €1.2 billion comes from Horizon Europe, the EU’s framework programme for research and innovation.
- €1 billion will come from the IHI industry partners
- €200 million will come from other life science industries or associations that decide to contribute to IHI as contributing partners.
Recipients of IHI funding
Any organisation established in the EU or a country associated to Horizon Europe is eligible to receive IHI funding. In practice, IHI funding primarily supports the participation in its projects of organisations like universities, research organisations, patient organisations, small and medium-sized enterprises (SMEs), and mid-sized companies. Depending on the type of call for proposals, larger companies may also be eligible to receive IHI funding. Details of who can receive funding is spelt out in the call texts.
Contributing to IHI: industry partners and contributing partners
Large companies that are members of the IHI industry partners contribute to the programme, primarily through ‘in-kind’ contributions (e.g. their researchers’ time, laboratories, data, compounds). They can also make cash contributions. IHI contributing partners provide resources to IHI in a similar way.
At least 45% of each project’s budget has to come from industry partner / contributing partner contributions.
Financial control
The IHI Programme Office manages the IHI programme as well as projects launched under the Innovative Medicines Initiative (IMI1 and IMI2). Half of the funding for IMI1, IMI2 and IHI comes from European tax payers, via the European research programmes, and we take the sound management of these funds extremely seriously.
EU funding is distributed mainly based on cost claims for work carried out in projects by organisations that are eligible to receive funding. Working on the principle that prevention is better than a cure, we provide project participants with information and guidance to help them avoid errors when submitting a claim.
In addition, our internal procedures and checklists are designed to ensure that when a cost claim is received from a project, errors are detected before payment is made. Finally, we send external auditors into project participants’ premises to go over cost claims with a fine-toothed comb. If they find that payments have been made in error, these are recovered.
Industry and other contributions to IMI / IHI
In public-private partnerships, the expected contributions from industry members and other contributors are described in the legislation and should match the contributions of the EU. All contributions are reported to the Programme Office. The reports are verified and progress is monitored to ensure we are on track to meet the contribution targets set out in the legislation.
Under IMI rules, EFPIA companies and IMI2 Associated Partners must report their contributions to IMI in a similar way to the cost claims of beneficiaries. The Programme Office carefully scrutinises these reports. All in-kind contributions declared must be accompanied by audit certificates, during or at the end of the project.
Contributions to IHI projects from industry members and contributing partners are managed in a similar way.
Industry and other Associated Partner (IMI2) or contributing partner (IHI) contributions are reported transparently in the annual accounts and Consolidated Annual Activity Reports.
Audits
The European Commission’s Internal Audit Service (IAS) is the official internal auditor for IHI and reports to our Executive Director and the Governing Board. The Executive Director draws assurance from the IAS audits and implements recommendations to update and improve our processes and procedures.
Another important level of financial control and assurance comes from the European Court of Auditors (ECA), which thoroughly scrutinises our accounts and activities. The ECA’s findings and recommendations are summarised in special annual reports. The reports, together with our responses to any issues raised, are published on the ECA website and in the Official Journal of the EU. We use these reports to further improve our processes and procedures.
European Parliament oversight
Finally, the European Parliament scrutinises how IHI has spent its money in a given year through the so-called ‘discharge procedure’. The lead committee here is the Budgetary Control (‘CONT’) committee, which analyses the ECA report and other materials such as joint undertakings’ consolidated Annual Activity Reports, Reports on Budgetary and Financial Management, and annual accounts. The CONT Committee’s opinion is compiled into a report which is voted on at the committee level before being passed to the Parliament plenary. The final decision on whether or not to grant discharge is taken by the plenary on the basis of the CONT report. Members of the Parliament may also include recommendations which we further use to improve our procedures and performance.